Explore lattice constants of boron carbides with PBN crucible
Introduction
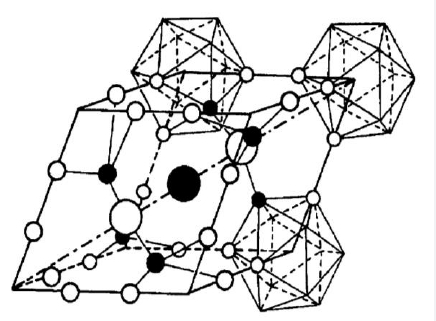
Boron carbide (B4C) is a fascinating compound known for its exceptional hardness, high melting point, and intriguing structural properties. Understanding the lattice constants of boron carbides is crucial for gaining insights into their structural characteristics and behavior. In a research article titled "Lattice Constants of Boron Carbides" by Terry L. Aselage, the author investigates the lattice constants of various boron carbide compositions. This study provides valuable data on the lattice constants of boron carbide samples. The work also sheds light on preparing boron carbide using loose powder in Pyrolytic Boron Nitride (PBN) crucibles.
Data Obtained and the Purpose of the Study
Terry L. Aselage conducted research to determine the lattice constants of different boron carbide compositions. These constants provide key insights about the distances between atoms and their arrangement in the crystal structure. By measuring the constants, researchers can learn about boron carbide's structural traits and potential applications.

PBN Parts
Preparation of Sample with Loose Powder in PBN Crucibles
To get accurate measurements, the samples were prepared using loose powder in PBN crucibles. Importantly, PBN crucibles are crucial because their unique traits minimize contamination.
In the preparation process, boron carbide powder was placed in PBN crucibles. Then, it was heated under specific reaction conditions and an inert atmosphere. This promoted a reaction between boron and carbon to form boron carbide crystals. Similarly, using PBN crucibles ensures the samples experience little contamination during synthesis. Specifically, the loose powder was subjected to controlled temperatures
Reasons for Using PBN Crucibles
PBN crucibles serve several important purposes when preparing boron carbide samples:
They prevent contamination because PBN offers exceptional chemical inertness. It does not react with the boron carbide sample. Since boron carbide contains boron, using a boron nitride crucible limits additional impurities. Moreover, nitrogen's volatile nature further reduces contamination during synthesis.
PBN crucibles maintain structural integrity and dimensional stability under extreme heat. Boron carbide synthesis involves high temperatures. This thermal stability ensures the crucible does not react with the sample or degrade during reactions, allowing reliable and reproducible results.
PBN ceramic materials suit boron carbide synthesis as they are compatible in boron-rich environments. The crucible material does not react with boron or boron carbide, keeping the sample's composition and crystal structure intact.
Conclusion
Terry L. Aselage's research provided insights into boron carbides' structural properties. Using loose powder in PBN crucibles enables synthesizing samples with minimal contamination.
By accurately determining lattice constants, researchers understand interatomic distances and crystal arrangement in boron carbide. This knowledge advances understanding of boron carbides and exploring applications in armor, nuclear technology, and cutting tools.
Selecting PBN crucibles for synthesis limits contamination, maintains high heat stability, and ensures compatibility in boron-rich conditions. With PBN crucibles, researchers can explore and utilize boron carbides' unique properties, advancing materials science and engineering.
